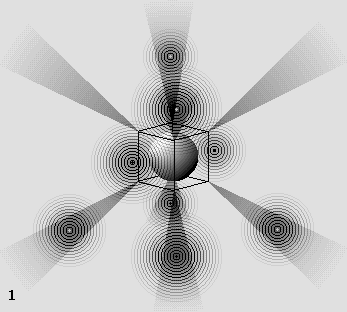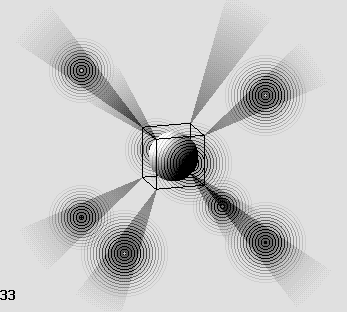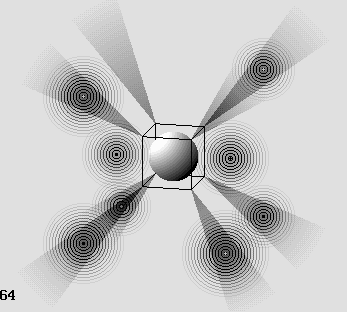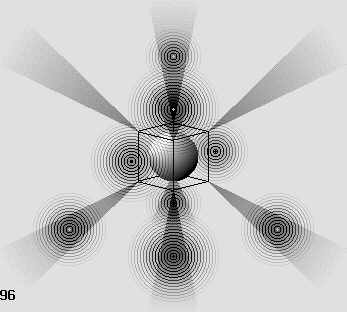
The oxygen atom.
Here, two shade cones are empty.
These cones can capture two electrons from one or two other atoms such as hydrogen or carbon.
|
This wave structure can explain a lot of phenomena, such as atomic shells, chemical bonding, electric current, semiconductors, etc. |
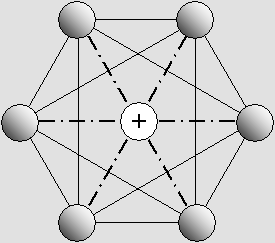
The six-electron structure looks like this along 8 different axes around the proton.
Those axes coincide with the center of 8 equilateral triangles which form the octahedron.
|
This wave structure shows that a proton radiates most of its energy along the 15 gluonic fields axes. This leaves 8 axes free from any radiation between them, producing 8 "shade cones" which look like this: |
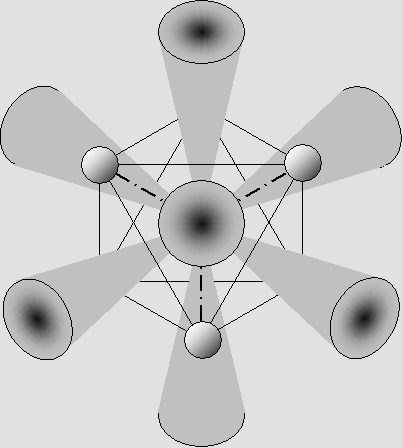
The shade cones can capture up to 8 electrons on the external atomic layer.
One or more electron can join two such systems while at least two cones coincide.
This explains chemical bonding.
|
Chemistry. A better knowledge of the proton wave diagram will certainly facilitate chemistry. Because of the Bohr atom model scientists had always thought that the electrons were rotating around the nucleus. The "electron clouds" from quantum mechanics had not tempered this in a convincing way. But today one can easily locate the appropriate places where the involved electrons should try to be. There is an obvious attraction effect between two ionized atoms, one positive and the other negative. The electron from the negative one will certainly try to fill one of the opposite and empty "black funnels". Then the two funnels involved will lock themselves together in order to be as close as possible as a reaction effect. Because those funnels are aligned on the 8 corners of a cube, up to 4 connections can be used in the same binding. This should make very strong molecules and crystalline structures. Moreover, there are other electrons inside the inner shells and smaller atoms may use those inner shells as well. It is a well known fact that lighter elements such as hydrogen, chlorine, sodium, carbon and oxygen are especially active. It seems obvious that inside molecules, the electron spin must alternate from one atom to another on the same axis. Otherwise a magnetic field would appear. This strongly indicates that any chemical reaction can be facilitated but it will be difficult to choose and alternate the correct spin. So the goal is to find the places where those electrons are most likely to fit. It should be very easy to construct complex molecules this way. More to come. Today I dare say that most of the puzzle pieces have been put together. This page will contain a lot of new information about the atom. If you are impatient, you may look at the French pages (33 full instead of 24 partially translated). |
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | You are here. | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 |
|
Gabriel LaFreniere Bois-des-Filion in Québec. absolu2000@hotmail.com On the Internet since September 2002. Last update September 26, 2007. |